On
our recent NZ speaking tour with Guy McPherson , I talked about how I
have witnessed the oceans dying over my 25 yr ocean sailing career
which has sadly come to an end. Robertscribbler explains exactly why
and the parallels with the Permian extinction we are now witnessing.
We've had a great run but it is now coming to an 'Abrupt' halt
"During
the Permian Extinction, such conditions, together with other impacts
of a global hothouse featuring a massive flood basalt, are thought to
have wiped out more than 70% of terrestrial organisms and a total of
more than 95% of all life on Earth."
---Kevin
Hester
Awakening
the Horrors of the Ancient Hothouse — Hydrogen Sulfide in the
World’s Warming Oceans
10
December, 2014
---Kevin Hester
“Dead
Cthulu waits dreaming…” H.P. Lovecraft
In
the 1930s, pulp horror writer H.P. Lovecraft penned tales of ancient
monsters called Old Ones that, if awakened, would emerge to devour
the world. One of these horrors, Cthulu, lay in death’s sleep in
his house called R’lyeh at the bottom of the Baltic Sea awaiting
some impetus to disturb him from necrotic slumber (ironically, the
Baltic sea bed contains one of the world’s highest concentrations
of the deadly hydrogen-sulfide producing bacteria that are a focus of
this article).
(2007 Hydrogen Sulfide emission off the coast of Namibia. Such emissions tend to color the surface water green and, in extreme cases, black. Image source: Earth Observatory)
In
the imaginary world of H.P. Lovecraft, terrible lore of these
horrific Old Ones, among which, Cthulu was the worst, lay stored in
ancient tomes. To learn of these mysteries was to risk madness. For
the Old Ones were too awful for the human mind to conceive without
succumbing to a hopeless darkness.
In
researching the terrors that could emerge in a world destabilized by
human warming, I am often reminded that human imagination is not
without a sense of dramatic irony. But in this case, the irony
invoked is that human imagining, in fiction, seems to sometimes
possess a broader perception of potential real world risks and their
implications for human thought, than the far more defined warning
signal coming from the sciences.
Cthulu,
in this case, may as well be a metaphor for one of the worst of the
world’s ancient climate horrors — the oceanic production of
hydrogen sulfide gas that occurred from time to time, during various
hothouse events. A production implicated in many of the worst mass
extinction events ever to mar the history of life on Earth.
Hydrogen
Sulfide — Bi-product of Bacterial Metabolism in the Ancient Oceans
In
understanding this ancient horror, we must first take a look at some
of the world’s oldest and smallest creatures. Primordial bacteria.
About
3.5 billion years ago, the Earth was a hot, toxic place, bombarded by
solar radiation. It was still cooling down after its initial
formation. The oceans had spilled out over its surface, but the
continents had yet to emerge. Atmospheric levels of CO2 were high and
oxygen was virtually nonexistent.
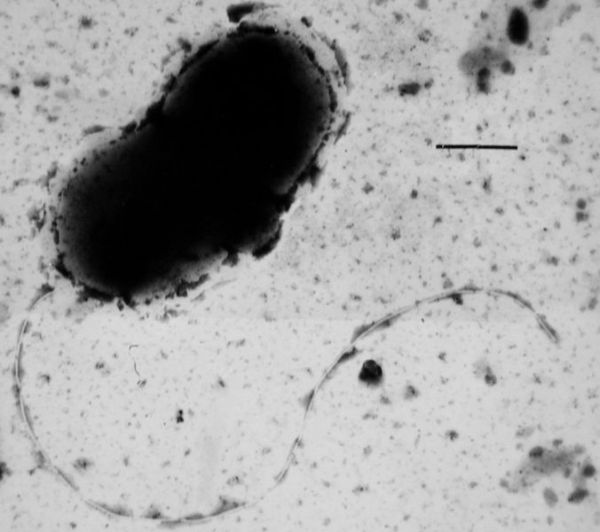
(Desulfovibrio vulgaris, one of the most well-researched hydrogen sulfide producing bacteria. Image source: Commons)
But,
in this world, small microbial organisms thrived. Deprived of oxygen,
which is the now typical means of respiration for non plant
organisms, the microbes required other sources for their simple
cellular metabolism. Sulphate was common in the world’s emerging
oceans and reacted well with hydrogen, which was also very common.
The result was the emergence of some of the oldest known living
organisms — the sulphate reducing bacteria.
Suphate
reducing bacteria combined sulphate and hydrogen to produce hydrogen
sulfide gas or H2S.
As
a result, ancient oceans were cauldrons bubbling over with hydrogen
sulfide which was the biproduct of these primordial organisms’
respiration in much the same way that oxygen is a biproduct of plant
respiration and CO2 is a biproduct of animal respiration. Such an
ocean state, called a Canfield Ocean by today’s scientists, was the
common state for the world’s oceans until the emergence of more
complex life around 2.5 billion years ago. By about 600 million years
ago, the Canfield Ocean state only very rarely came into being and
when it did, mass death tended to rapidly follow.
Changes
Came With the Emergence of Oxygen
As
the Earth system matured and new organisms came into being, CO2
reducing photosynthetic life emerged and began to produce an
abundance of oxygen. Toxic to the ancient organisms, the abundance of
oxygen pushed the sulphate reducing bacteria into the world’s
low-oxygen corners. The deep ocean, or anaerobic mud became a haven
for these tiny primordial monsters. Never again would they dominate
as they once did. But, from time to time, when priomordial ocean
states would infrequently emerge during various hot-house phases in
Earth’s climate progression, these life forms would explode,
producing prodigious volumes of what, to more complex life, was the
very toxic hydrogen sulfide gas.
A
Toxic, Volatile Gas
Hydrogen
sulfide is directly toxic to most plant and animal based life. Its
effects in animals are similar to that of hydrogen cyanide in that it
eventually results in cardio-pulminary shock and then death. Lower
levels of hydrogen sulfide are associated with loss of smell,
blindness, respiratory infections, and loss of neurological and
nervous system function. At very low levels, hydrogen sulfide is non
toxic and is even produced in cells to perform various functions.
Human lethality begins at around 600 parts per million. Smaller
mammals with higher respiration rates begin to show lethality at
around 450 ppm. Doses in the range of 10-20 parts per million have
been known to cause eye irritation and damage over long periods of
exposure. Levels over 50 ppm are generally considered harmful if
exposure occurs for long durations. Doses between the irritation dose
(10 ppm) and the lethality dose (600 ppm) over extended periods are
shown to cause the eye damage and degenerative nerve and lung changes
listed above.
In
the environment, hydrogen sulfide causes numerous other damaging
impacts. The gas reacts with hydroxyl and oxygen over the course of
about 1 to 3 days to produce sulfur dioxide. Aside from providing a
mechanism to draw down local oxygen levels, the sulfur dioxide
product can end in the stratosphere where it substantially degrades
the protective ozone layer.
Though
hydrogen sulfide is slightly heavier than air, tending to pool at
lower elevations, it is light enough to be born aloft by winds to
various layers of the atmosphere and its even lighter sulfur dioxide
products are quite a bit more mobile. At high enough atmospheric
concentrations, both it and its sulfur products could begin to
seriously degrade the Earth’s protective ozone layer. And evidence
exists in the geological record of such events occurring on at least
a couple of occasions during the last 250 million years. Notably,
during the Permian extinction event, large numbers of fossils have
been found with the characteristic UV damage that would occur in a
world in which the ozone layer had been greatly degraded.
At
high enough concentrations, hydrogen sulfide is volatile enough to
burn. A 4.3 percent concentration is immediately combustible,
producing a bluish flame. This extraordinarily high concentration
would be almost immediately lethal to humans if inhaled and usually
only presents a fire risk at highly concentrated sources.
In
the current day, high concentrations of hydrogen sulfide gas are
often associated with natural gas extraction. Natural gas, by volume,
can contain as much as 90 percent hydrogen sulfide. The hydrogen
sulfide, in this case, occurs due to catalytic reaction of the
hydrocarbon with certain minerals present in the Earth. Though not
produced by the same mechanisms as oceanic hydrogen sulfide, the gas
in this form is just as dangerous and is a constant concern to
workers of the oil and gas industry. Notably, risks of hydrogen
sulfide exposure, leaks, and release into the environment have
greatly increased with the widespread adoption of hydro-fracking
practices that use high pressure liquids to rupture tight gas
deposits and chaotically release the substance for its collection at
one of the US’s 1 million well sites.
In
general, the volatility, danger, and toxicity of the gas is difficult
to overestimate. Notably, its lethality resulted in its use as a
chemical weapon during World War I.
Culprit
of Past Mass Extinctions
High
concentrations of hydrogen sulfide, resulting both from its
production in a Canfield type ocean state and, possibly, through its
release in large methane pulses from the sea bed during catastrophic
warming events, has been implicated in numerous mass extinction
events both on land and in the ocean. Notably, the Permian-Triassic
extinction, the Triassic-Jurassic extinction, and the PETM extinction
in the deep oceans all show signs related to ocean anoxia and varying
levels of hydrogen sulfide gas production. Earlier mass extinctions
such as the Devonian and Ordovician extinctions were also likely
caused by anoxia and related hydrogen sulfide production. Lesser
extinctions in which ocean anoxia also probably played a part
include the Ireviken, Mulde, Lau, Toarcian and
Cenomanian-Turonian events.
Prominent
researchers such as Ward and Kump propose that hydrogen sulfide
production by sulfate reducing bacteria is a primary extinction
mechanism in stratified and anoxic oceans due to their inevitable
multiplication in these environments which are, to them, far more
favorable than oxygen-rich mixed oceans. In a Canfield Ocean world,
large, episodic releases of hydrogen sulfide gas would cause local
mass poisonings of land dwelling animals, especially of those living
near large ocean-linked bodies of water. The ocean itself would be
brimming full and spilling over with this nasty substance. This
condition would be highly toxic to most life, requiring extreme
adaptation to survive in naturally occurring havens.
Separate
depletion of atmospheric oxygen through both the plant killing
mechanism of hydrogen sulfide gas and its long-term reaction with
oxygen would also make life far more difficult to terrestrial
creatures. Finally, the massive amounts of sulfur dioxide produced in
such a world would combine with the hydrogen sulfide pulsing into the
atmosphere to create an ongoing, long-term degradation of the ozone
layer, further harming surface dwelling plants and animals.
During
the Permian Extinction, such conditions, together with other impacts
of a global hothouse featuring a massive flood basalt, are thought to
have wiped out more than 70% of terrestrial organisms and a total of
more than 95% of all life on Earth.
Occurrence
in Current Seas
(Expanding bottom anoxia, hypoxia and hydrogen sulfide production since 1960 in the bottom zone of the Baltic Sea. Red indicates region experiencing low or no oxygen content. Black indicates areas where H2S gas is detected. Image source: Baltic Sea Trends)
The
world’s oceans, according to recent research, are rapidly becoming
more stratified and less oxygen-rich. The result is that mixing
between various layers of the ocean is beginning to shut down
reducing oxygen content in the deep ocean and spurring the expansion
of numerous oceanic dead zones.
Over
the past 150 years, the Pacific Ocean was observed to become more
stratified at a pace ten times that seen during the end of the last
ice age about 12,000 years ago. Such a rapid pace of stratification
is putting severe stress on the world’s oceans with numerous
regions showing the effects of low oxygen (hypoxia) and some regions
succumbing to increasingly anoxic states.
These
low oxygen events have been associated with multiplying oceanic dead
zones. Very large dead zones have been observed in the Pacific,
specifically off the coast of Oregon. Other major dead zones continue
to be observed at the mouth of major river systems, such as within
the Gulf of Mexico, where the appearance of massive related toxic
algae blooms is now an almost annual event. In general, almost all
ocean dead zones are expanding leading to the dramatic reduction in
habitat size of numerous fish species. And even the most cursory
research provides ample evidence that ocean hypoxia is expanding
concurrently with a rapidly expanding ocean stratification.
When
combined with the jarring effects of rapid ocean warming and
expanding acidification, it becomes plainly obvious to almost any
ocean ecologist that the world’s ocean system is suffering the
heavy bombardment of a new mass extinction event.
It
is this kind of low or no oxygen environment that is a prime breeding
ground for hydrogen sulfide producing bacteria. In numerous places
around the world, such as off the coast of Namibia, in the Black Sea,
in the Baltic Sea, in the Gulf of Mexico, in the Chesapeake Bay, and
off the coast of Oregon, large and expanding zones of hydrogen
sulfide have been observed in deep water environments. In some
regions, this hydrogen sulfide occasionally penetrates to the surface
layer resulting in major fish kills and a concordant rotten egg
smell.
Off
the Oregon coast, in perhaps one of the most extreme examples of
ongoing ocean hypoxia, one of the world’s largest and most
oxygen-starved dead zones continues to expand. The oxygen levels in
this region are so low that local fisherman often bring back horrific
tales of baby bottom dwelling creatures such as crabs and octopus
climbing anchor ropes to escape the dangers of their oxygen-starved
environment. In another, possibly related event, masses of starfish
perished during 2013 and 2014 as they, over the course of a few
weeks, turned to goo. The fact that this sci-fi esque mass death of
starfish occurred near one of the world’s largest dead zones should
not be lost on those concerned for world ocean health.
But
perhaps even more concerning is the fact that this region off the
Oregon coast is producing substantial volumes of hydrogen sulfide
gas. Volumes high enough in concentration to occasionally cross the
ocean-air boundary.
Oregon
possesses numerous features that would aid in the transport of this
gas to the surface. Primarily, the near Oregon ocean system
frequently features strong up-welling currents. These currents can
push bottom waters through stratified layers and cause them to
contact the surface. If these oxygen starved bottom waters contain
hydrogen sulfide gas, as they increasingly do, this harmful gas can
be transported into the local atmosphere through mixing.
Such
events, thus far, have been limited. However, since the Oregon dead
zone’s discovery in 2001, its expansion has been both deeply
concerning and well documented, showing a rapid and dangerous growth
over the 13 years since its emergence. Despite the documented
expansion of deep water hydrogen sulfide in numerous oceanic regions,
the only other ocean zone on Earth observed to emit hydrogen sulfide
gas to the atmosphere is in the region of coastal Namibia.
In
Namibia, huge volumes of organic compounds fall into the sea after
being flushed down ocean terminating streams and rivers. These
organic compounds rain down into the deep ocean directly off Nambia’s
coasts. There, the ocean bottom hosts both an anoxic environment and
masses of hydrogen sulfide producing bacteria. As a result, toxic
hydrogen sulfide gas periodically erupts from the ocean and into the
atmosphere there.
The
Very Real Threat That is Oceanic Hydrogen Sulfide Gas Production
There
are few limiters to oceanic hydrogen sulfide production in the
world’s increasingly stratified and oxygen starved oceans.
Sulphate, which the bacteria require for respiration, is one of the
most common ocean elements. In the current ocean, it is present in
volumes greater than those seen during the Permian Extinction when
these tiny monsters are thought to have done their worst.
Iron
and manganese in the world ocean system aids in the development of
less permeable boundary layers that help keep a lid on deep ocean
concentrations of hydrogen sulfide. However, even in the anemic
circulation of stratified and Canfield oceans, upwelling will bring
the gas to the surface in certain regions. In addition, as the oceans
contain greater and greater volumes of the toxic gas, it will push
closer and closer to the surface, rendering metals that help
reinforce the boundary layer a practically useless prophylactic (such
high metal concentrations currently prevent hydrogen sulfide from
penetrating the surface layer in the Black and Baltic Seas as well as
in the Chesapeake Bay).
In
addition, modern industrial farming practices provide extra nutrients
upon which these dangerous microbes can feed. High levels of hydrogen
sulfide in the deeper regions of the Chesapeake Bay, for example,
owes its existence, in part, to massive farm run-off into the Bay and
the dumping of mass volumes of nutrients upon which the sulphate
reducing bacteria can feed.
It
is important to note that we observe heightened levels of hydrogen
sulfide gas in the world ocean system now. As hypoxia and anoxia
progress with the human-caused warming of the oceans, and as glacial
melt interrupts and alters the now strong ocean currents and related
mixing, it is certain that hydrogen sulfide production in the deep
ocean will continue to increase resulting in elevating levels of harm
to ocean dwelling animals and ever more numerous instances of
hydrogen sulfide gas contact with coastal and surface waters.
Dead
Cthulu Riss
In
the context of increasing ocean hypoxia and stratification, we might
do well to remember that we are tiny, weak beings at the mercy of
great natural forces which we can barely conceive or understand.
Forces that we have unwittingly, callously and ignorantly set into
motion.
*
* * * *
Long
ago, when I was a ten year old child, I was fortunate enough to meet
an amazingly kind, adventurous and inquisitive man. The man, whom I
will call Rick to keep safe his identity, was a bit of a local
paramour in ocean and bay research. He was constantly in contact with
both the ocean and adjacent Chesapeake bays, ever venturing out to
explore and to conduct research on marine life. In later years, he
would be the impetus behind annual summer marine science camps hosted
by the Virginia Institutes of Marine Science, Norfolk Academy, and
Old Dominion University. But this was later. Now, Rick was helping an
elementary school student present on the issue of our then expanding
understanding of marine science.
Living
so close to the bay and ocean, I was intimately in contact with the
living boundary of land and sea. In the more demanding and less
stimulating forum that was public education, I seldom had the
opportunity to indulge my passion for the oceans. But at age 10 I was
given the opportunity to give a broad marine science presentation for
my classmates. As part of my project, I constructed posters and
models depicting the current state of world ocean research. I
graphically illustrated the various known zones of the bathysphere,
the light and life filled ones and the more mysterious and far less
well understood depths. But Rick was the centerpiece of my
presentation. He was my keynote. And he energetically answered all my
own and fellow students’ questions, speaking in the kind and
intriguing manner that would later draw so many into his charismatic
orbit.
In
later years, I would attend Rick’s summer marine science camps on
two different occasions. In both cases, I observed what appeared to
be an increasing concern about both the health of the Chesapeake Bay
and the neighboring oceans. In later years, Rick’s attitude, once
so full of optimism, bordered on cynicism. The world he loved so
deeply was experiencing death on a scale that horrified him. And he
harbored a deep sense of betrayal that we weren’t doing more to
stop the senseless slaughter of so many of the living things he saw
as both beautiful and wondrous.
In
the mid 2000s, Rick committed suicide. To me, one of the great ocean
pioneers of my developmental years had passed away by taking his own
life. And I couldn’t help but wonder if the horrible ways in which
the oceans that he so loved were changing was just too much for him.
If the commercialization and cheapening of all the things he held
most dear along with their subsequent damaging and putting at great
risk of terrible harm had robbed his life of beauty and purpose.
Rick
was, if anything, a very intelligent and sensitive man. He knew what
was happening to the Bay and ocean on a personal level. When the Bay
was harmed it was as if it hurt Rick too.
Rick
also knew how temperature changes affected the depths. For he was on
the front line studying it. He was hauling up the fish and the water
samples. He was doing the measuring with his own hands.
Was
the awakening of terrible Cthulu, in the form of hypoxia, anoxia and
deadly hydrogen sulfide producing bacteria, too much for Rick to
continue bearing mute witness? Did his pleas to those working in the
marine science community fall only on deaf ears? Was it just too much
for this sensitive, feeling, and intelligent man to bear?
*
* * * *
If
Rick taught me anything it was that our lives and the life of the
ocean are deeply connected. One cannot remain healthy without the
other. In contrast to this basic understanding, the damage our
continued industrial emission of greenhouse gasses is doing to the
world ocean system is a horrific travesty. And the damage we have
already caused, have already done to those most sensitive creatures
among us, have already set in play for future decades and centuries,
is tremendous.
The
ocean suffocates, bleeding deadly hydrogen sulfide gas. Cthulu rises
from his ancient house in the depths. And yet we still continue down
the wretched path in pursuit of more terrible things to come.
Links:





No comments:
Post a Comment
Note: only a member of this blog may post a comment.