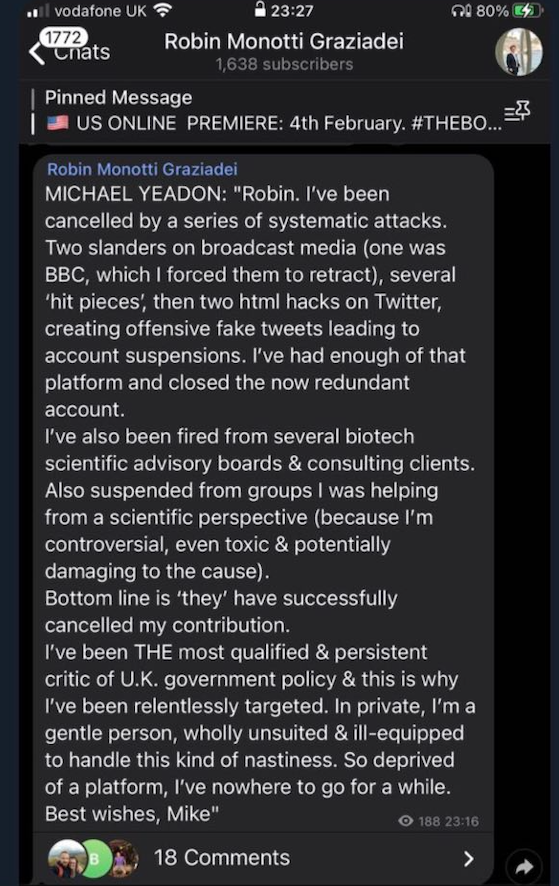
Yardley Yeadon is one of the best-qualified and decent people in the biotech industry but has been subject to a campaign of vilification and disinformation because he has dared to speak out.
Has Twitter closed the account of Yardley Yeadon?
Indeed he is a gentle person, unsuited and ill-equipped to handle this sort of nastiness thrown at him for simply speaking the truth.
Hear him talk for himself
Dr. Yeadon has been subject to a campaign of vilification and disinformation for speaking out on this.
Covid Vaccine is Female Sterilization – Head of Pfizer Research
Article by Health & Money News.
The vaccine contains a spike protein (see image) called syncytin-1, vital for the formation of human placenta in women. If the vaccine works so that we form an immune response AGAINST the spike protein, we are also training the female body to attack syncytin-1, which could lead to infertility in women of an unspecified duration.

Dr. Wodarg and Dr. Yeadon request a stop of all corona vaccination studies and call for co-signing the petition
2020NEWS at Health and Money News
On December 1, 2020, the ex-Pfizer head of respiratory research Dr. Michael Yeadon and the lung specialist and former head of the public health department Dr. Wolfgang Wodarg filed an application with the EMA, the European Medicine Agency responsible for EU-wide drug approval, for the immediate suspension of all SARS CoV 2 vaccine studies, in particular the BioNtech/Pfizer study on BNT162b (EudraCT number 2020-002641-42).
Dr. Wodarg and Dr. Yeadon demand that the studies – for the protection of the life and health of the volunteers – should not be continued until a study design is available that is suitable to address the significant safety concerns expressed by an increasing number of renowned scientists against the vaccine and the study design.StarGate TV Series Warned Us In 2001 About the
Vaccination Disaster Facing Us Today
On the one hand, the petitioners demand that, due to the known lack of accuracy of the PCR test in a serious study, a so-called Sanger sequencing must be used. This is the only way to make reliable statements on the effectiveness of a vaccine against Covid-19. On the basis of the many different PCR tests of highly varying quality, neither the risk of disease nor a possible vaccine benefit can be determined with the necessary certainty, which is why testing the vaccine on humans is unethical per se.
Furthermore, they demand that it must be excluded, e.g. by means of animal experiments, that risks already known from previous studies, which partly originate from the nature of the corona viruses, can be realized. The concerns are directed in particular to the following points:
- The formation of so-called “non-neutralizing antibodies” can lead to an exaggerated immune reaction, especially when the test person is confronted with the real, “wild” virus after vaccination. This so-called antibody-dependent amplification, ADE, has long been known from experiments with corona vaccines in cats, for example. In the course of these studies all cats that initially tolerated the vaccination well died after catching the wild virus.
- The vaccinations are expected to produce antibodies against spike proteins of SARS-CoV-2. However, spike proteins also contain syncytin-homologous proteins, which are essential for the formation of the placenta in mammals such as humans. It must be absolutely ruled out that a vaccine against SARS-CoV-2 could trigger an immune reaction against syncytin-1, as otherwise infertility of indefinite duration could result in vaccinated women.
- The mRNA vaccines from BioNTech/Pfizer contain polyethylene glycol (PEG). 70% of people develop antibodies against this substance – this means that many people can develop allergic, potentially fatal reactions to the vaccination.
- The much too short duration of the study does not allow a realistic estimation of the late effects. As in the narcolepsy cases after the swine flu vaccination, millions of healthy people would be exposed to an unacceptable risk if an emergency approval were to be granted and the possibility of observing the late effects of the vaccination were to follow. Nevertheless, BioNTech/Pfizer apparently submitted an application for emergency approval on December 1, 2020.
CALL FOR HELP: Dr. Wodarg and Dr. Yeadon ask as many EU citizens as possible to co-sign their petition by sending the e-mail prepared here to the EMA.







No comments:
Post a Comment
Note: only a member of this blog may post a comment.